
About IMAAVY

IMAAVY is the first and only fully human FcRn-blocking mAb designed for long-lasting, targeted binding.1-5

The high-affinity and high-specificity binding of IMAAVY to FcRn enables long-lasting target engagement both inside and outside the cell.1,2,6

This enables the reduction of circulating IgG levels, including those of pathogenic IgG.1,2*
*
In the phase 3 pivotal study, the pharmacological effect of IMAAVY was assessed by measuring the decrease in serum IgG levels and anti-AChR and anti-MuSK autoantibody levels. In patients positive for anti-AChR and anti-MuSK autoantibodies who were treated with IMAAVY, there was a reduction in anti-AChR and anti-MuSK autoantibodies relative to baseline. Decreases in total IgG levels followed a similar pattern.1
IgG reduction
IgG reduction over time with IMAAVY2
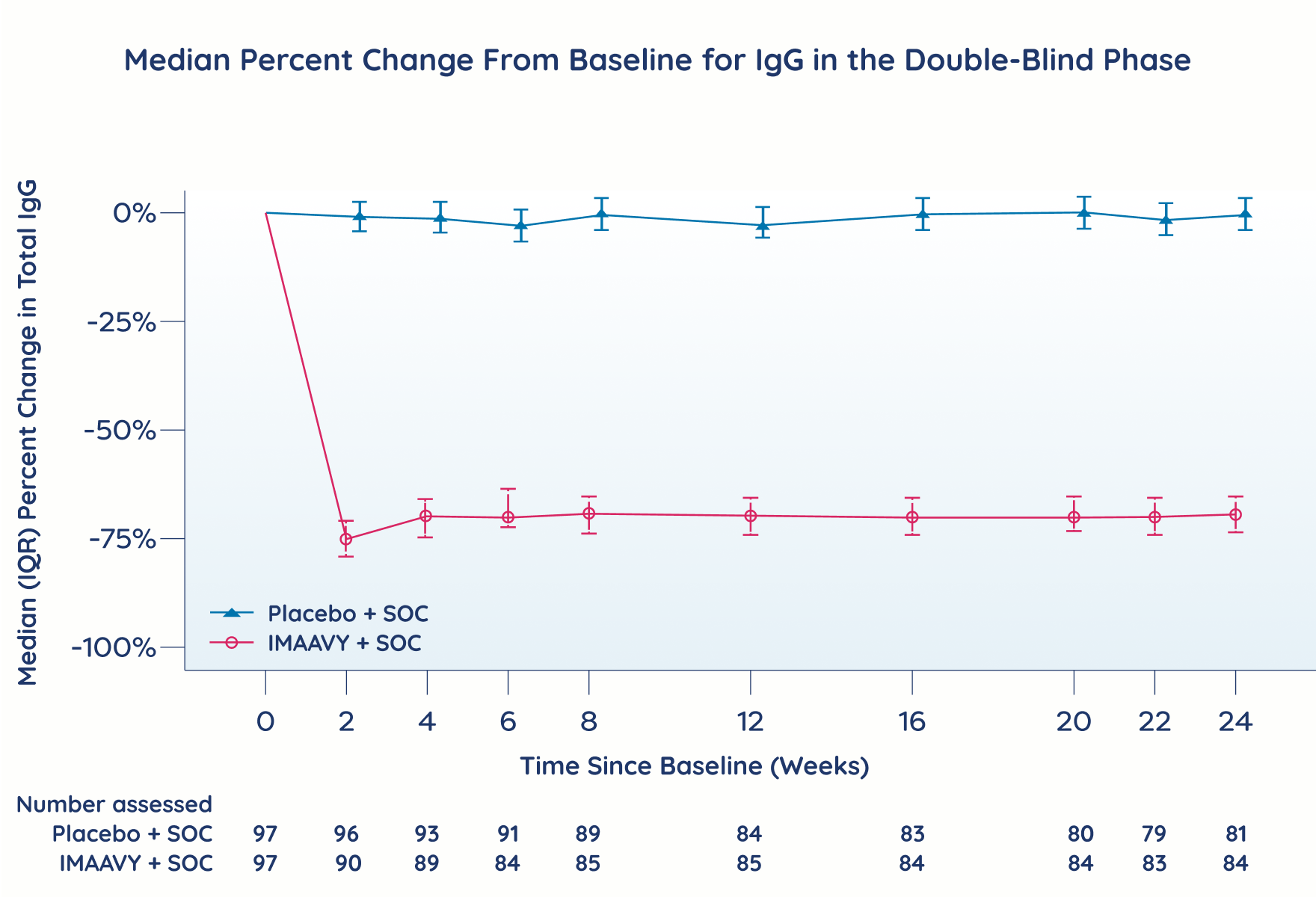
The clinical significance of these data has not been established, and comparisons cannot be made.
AChR=acetylcholine receptor; FcRn=neonatal fragment crystallizable receptor; gMG=generalized myasthenia gravis; IgG=immunoglobulin G; IQR=interquartile range; MOA=mechanism of action; MuSK=muscle-specific tyrosine kinase; SOC=standard of care.
FAQs
Frequently asked questions about the IMAAVY MOA
A fully human design can help to minimize immune reactions by resembling natural human proteins.7
In the phase 3 pivotal study, the pharmacological effect of IMAAVY was assessed by measuring the decrease in serum IgG levels and anti-AChR and anti-MuSK autoantibody levels. In patients positive for anti-AChR and anti-MuSK autoantibodies who were treated with IMAAVY, there was a reduction in anti-AChR and anti-MuSK autoantibodies relative to baseline. Decreases in total IgG levels followed a similar pattern.1
IMAAVY does not impact IgG production; it reduces circulating IgG by binding FcRn and preventing IgG recycling. In the phase 3 pivotal study, the pharmacological effect of IMAAVY was assessed by measuring the decrease in serum IgG levels and anti-AChR and anti-MuSK autoantibody levels. In patients positive for anti-AChR and anti-MuSK autoantibodies who were treated with IMAAVY, there was a reduction in anti-AChR and anti-MuSK autoantibodies relative to baseline. Decreases in total IgG levels followed a similar pattern.1
IMAAVY binds to FcRn with high specificity and high affinity at both neutral (extracellular) and acidic (intracellular) pH levels, resulting in the reduction of circulating IgG levels including those of pathogenic IgG.6
IMAAVY demonstrated a reduction only in immunoglobulin G (IgG).1




Once you’ve made the decision
to prescribe IMAAVY™
Sign your patients up for support to help begin their IMAAVY™ journey


