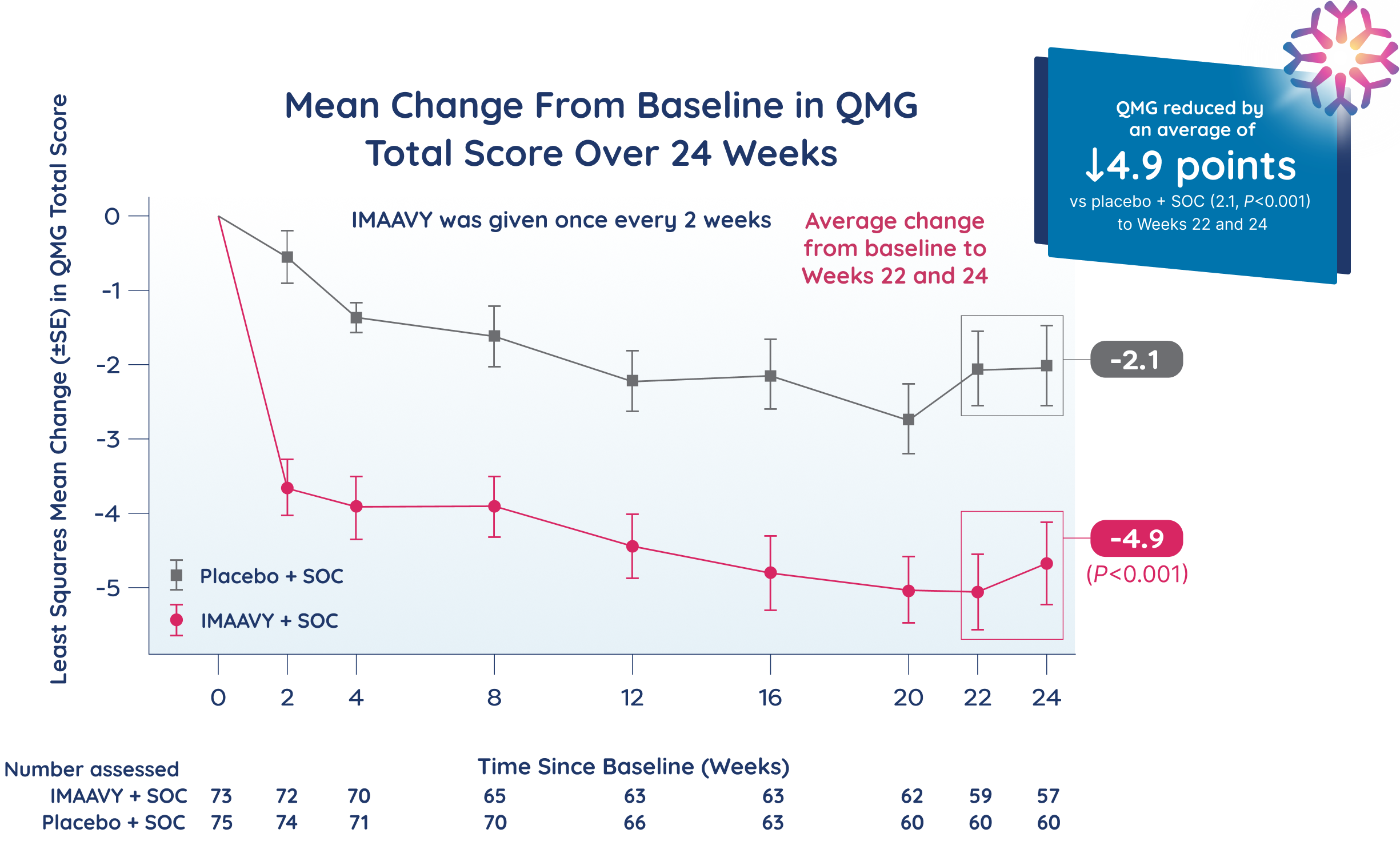
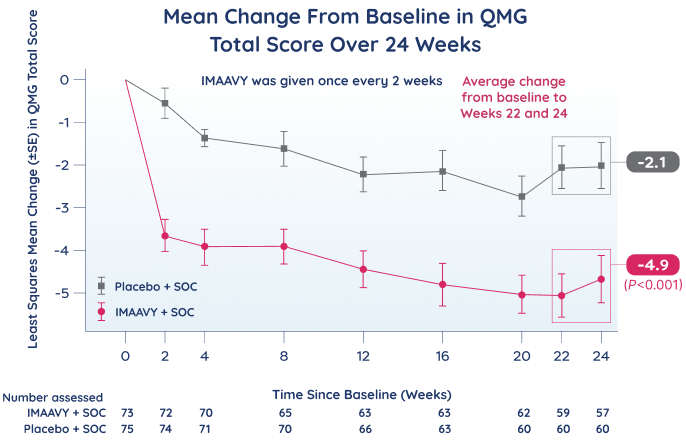
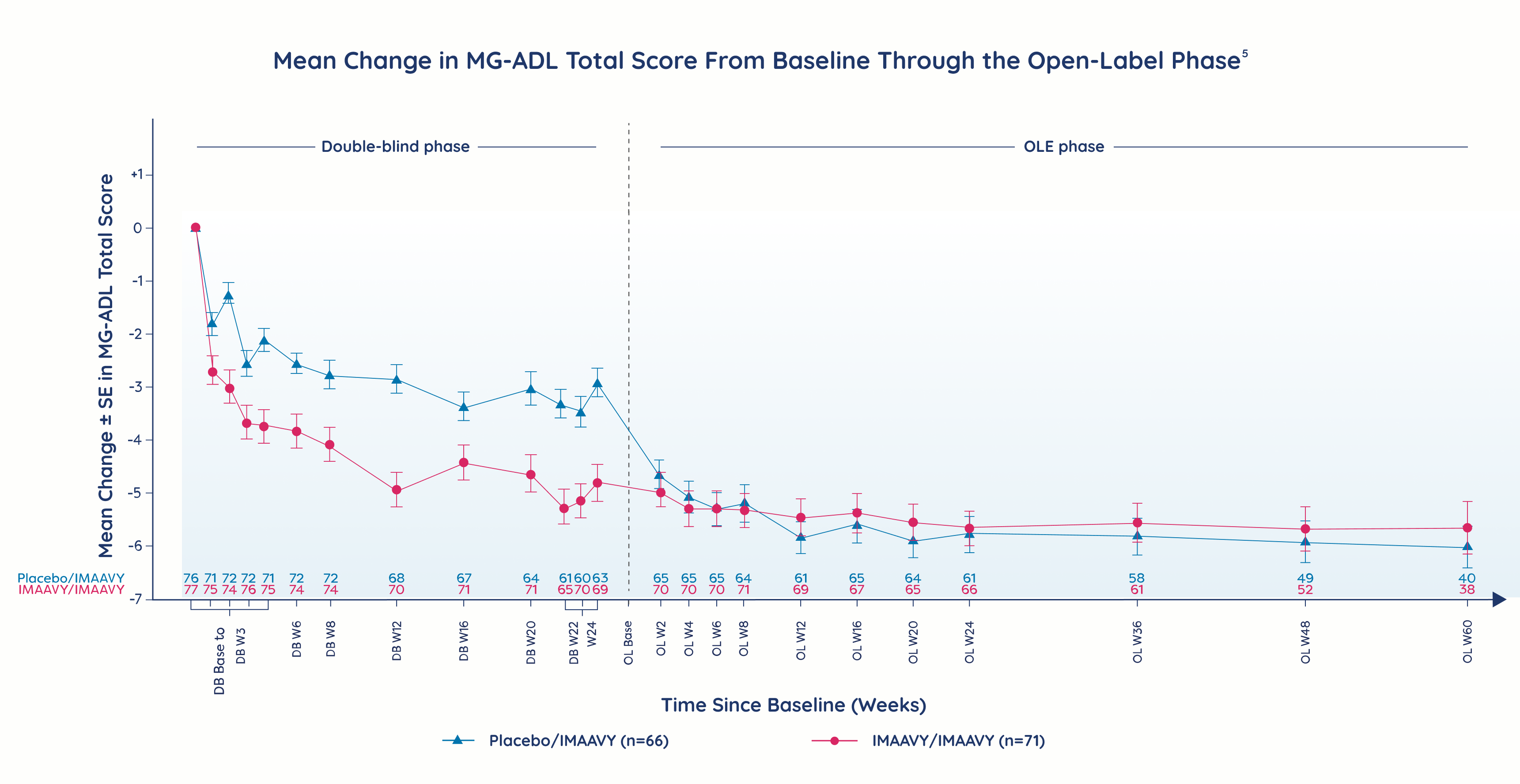
Primary endpoint
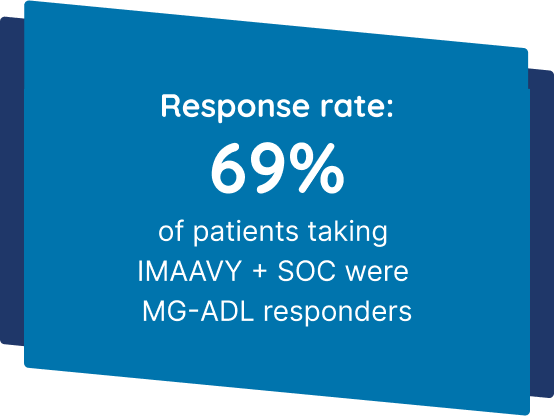
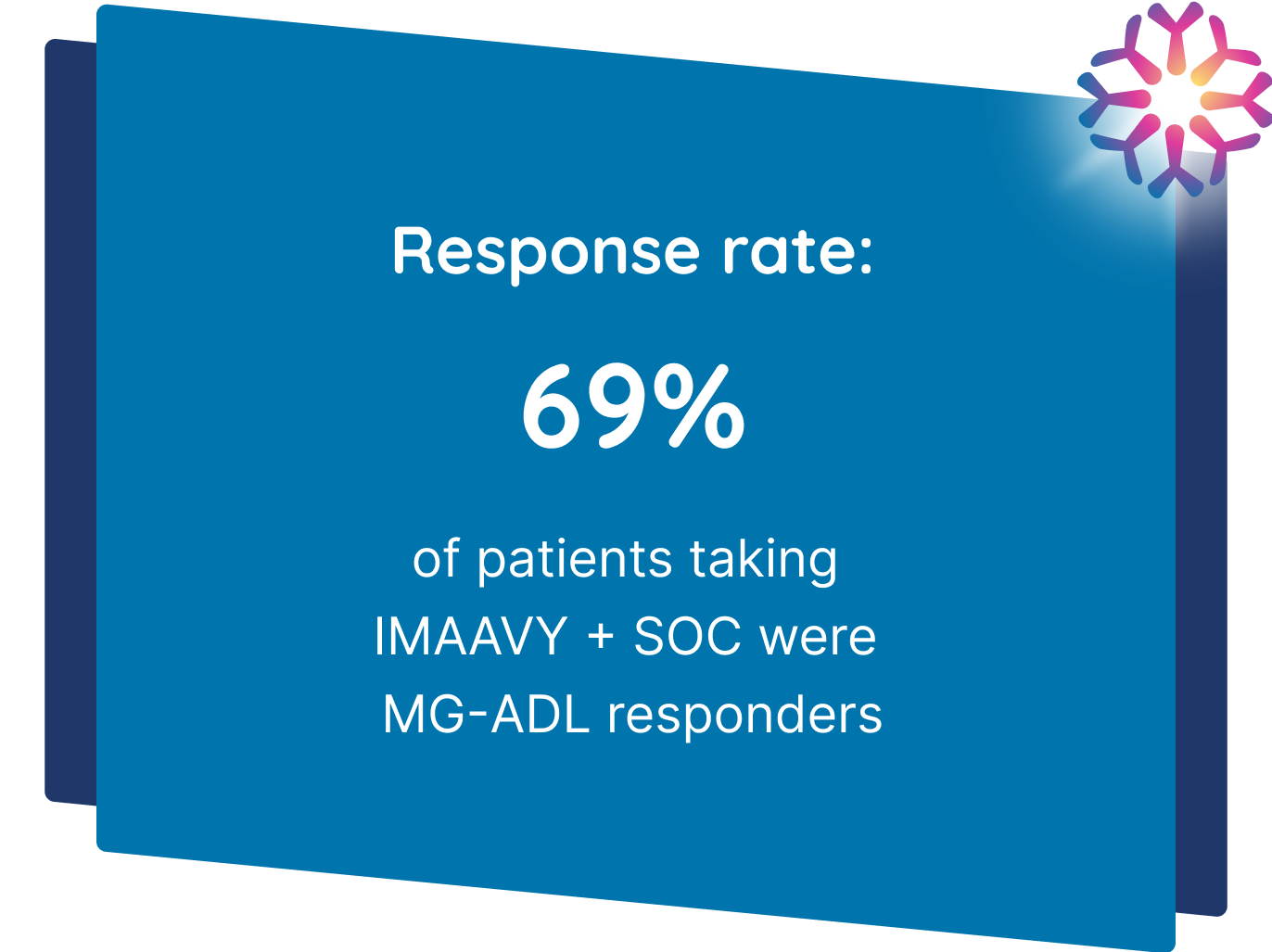
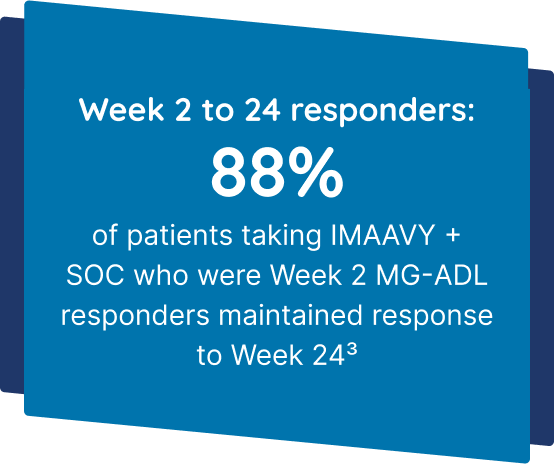
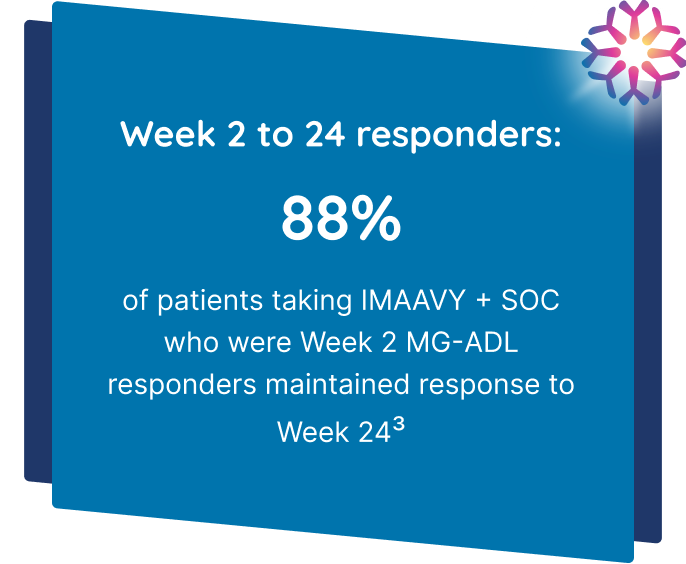
IMAAVY + SOC demonstrated superior disease control† that didn’t fade1,2
vs placebo + SOC through Week 24
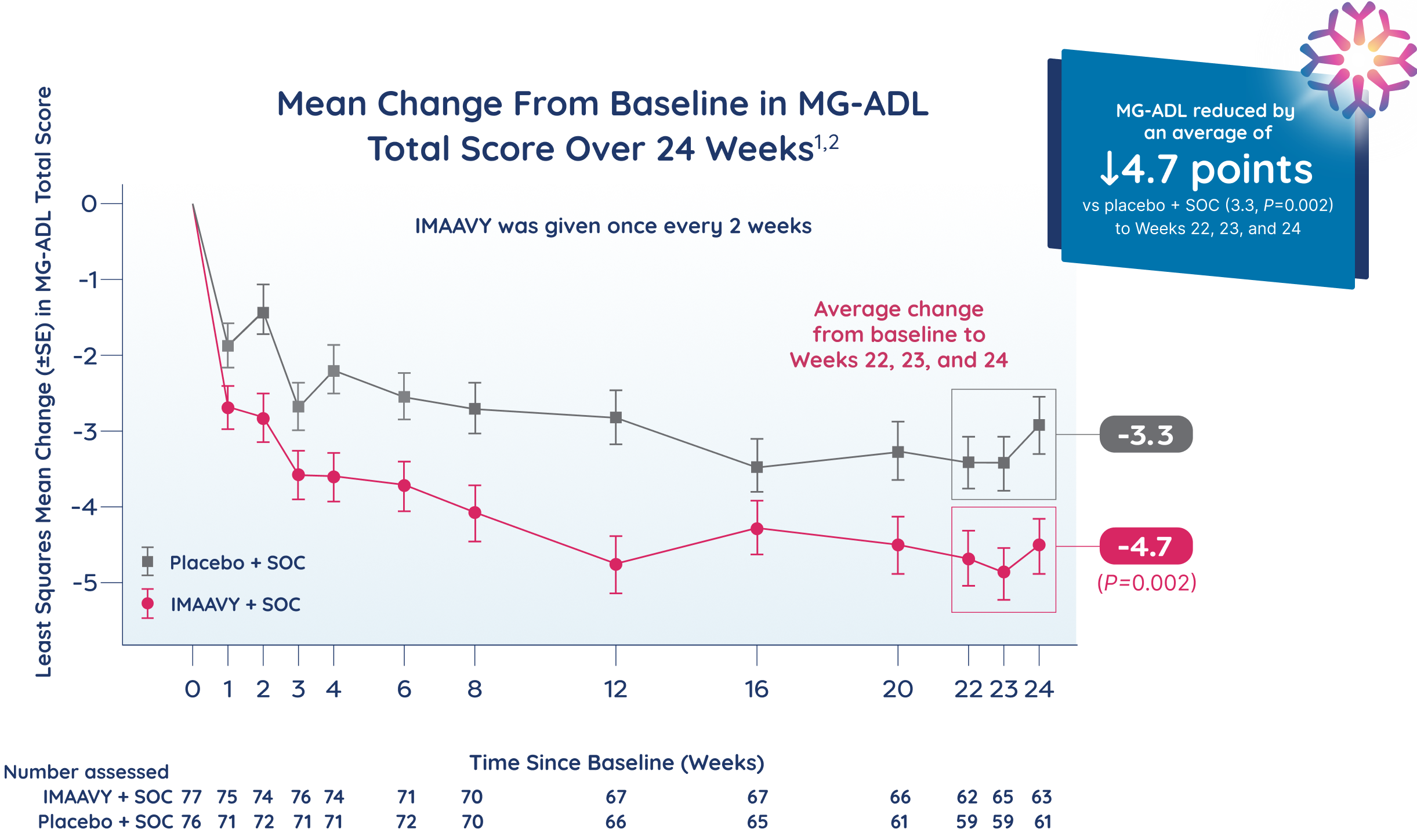
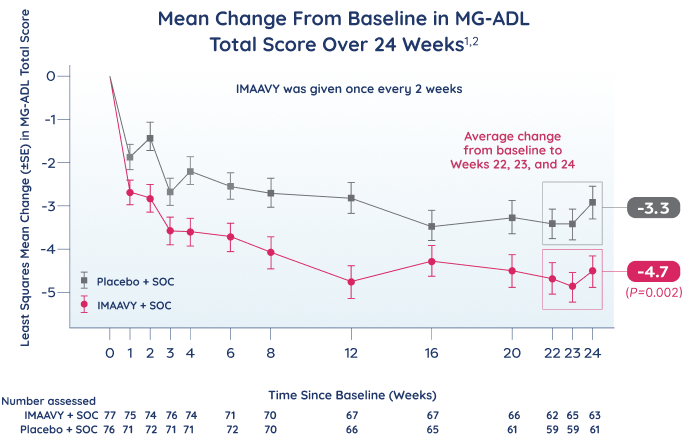
Patients taking IMAAVY + SOC therapy experienced a 4.7-point mean improvement in total MG-ADL score to Weeks 22, 23, and 24 while patients taking placebo + SOC therapy saw a 3.3-point mean improvement (P=0.002).1,2
†As measured by mean change from baseline to Weeks 22, 23, and 24 in MG-ADL total score.